FAQ 8 - General Corrosion Resistance: The Normal State for Stainless
Stainless steels resist corrosion because they have a self-repairing "passive" oxide film on the surface. As long as there is sufficient oxygen to maintain this film and provided that the level of corrosives is below the steel's capacity of the particular material to repair itself, no corrosion occurs. If there is too high a level of (say) chlorides, pitting occurs. As an example, 316 works well is tap water (<250 ppm) all over Australia, but will rapidly corrode in seawater because seawater has very high chloride levels (20,000 ppm).
Download ASSDA Technical FAQ 8 (PDF)
If there is not enough oxygen and the local corrosives are not high enough to cause pitting, then general corrosion can occur. This might happen in a crevice (which has very limited oxygen) or in a strong, reducing acid (such as mid concentrations of sulphuric acid). General corrosion can occur when there are stray currents flowing from stainless steel to ground. This can happen in mineral extraction if the bonding arrangements are inadequate during electrowinning. General corrosion may also occur from galvanic effects, e.g. if a conductive carbon gasket is used on stainless steel in an aggressive environment.
QUANTIFYING CORROSION RESISTANCE
For circumstances where general corrosion is expected, graphs are available called iso-corrosion curves. They plot the effect of a single chemical and corrosion rate for temperature against concentration. An example is the graph below of a 42% nickel alloy 825 in pure sulphuric acid with air access. This graph shows that the corrosion rate increases with temperature and that provided the temperature is less then ~45 °C and a corrosion rate of 0.13 mm/year is acceptable, alloy 825 would be suitable for any concentration of pure sulphuric acid. The boiling point curve is often included to show the limits of data at atmospheric pressure.
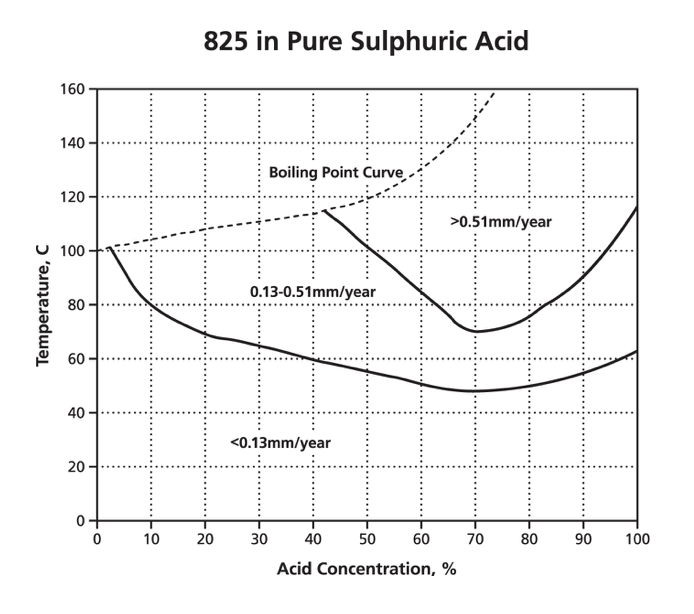
Most of the following graphs are from Outokumpu's Corrosion Handbook. The specific alloy compositions are tabulated in that Handbook and in the Appendix of the ASSDA Technical FAQ 8.
However, a series of graphs each showing the results for one material over the full range of concentrations and temperatures is cumbersome and so multi-material plots are used for the initial material selection. Titanium is frequently included because of the widespread expectation that it is the “super” solution – although the data shows this is not always correct.
The two graphs below show data for austenitic and duplex stainless grades in pure sulphuric acid. However, only the 0.1 mm/year lines are drawn for each alloy because it is assumed that a loss of 0.1 mm/year would be acceptable for continuous exposure during 365 days per year. This assumption may not be acceptable if, for example, the process using the acid required very low iron levels. For each material, the temperature and concentrations of pure sulphuric acid that are below the line would mean a corrosion rate of less than 0.1 mm/year.
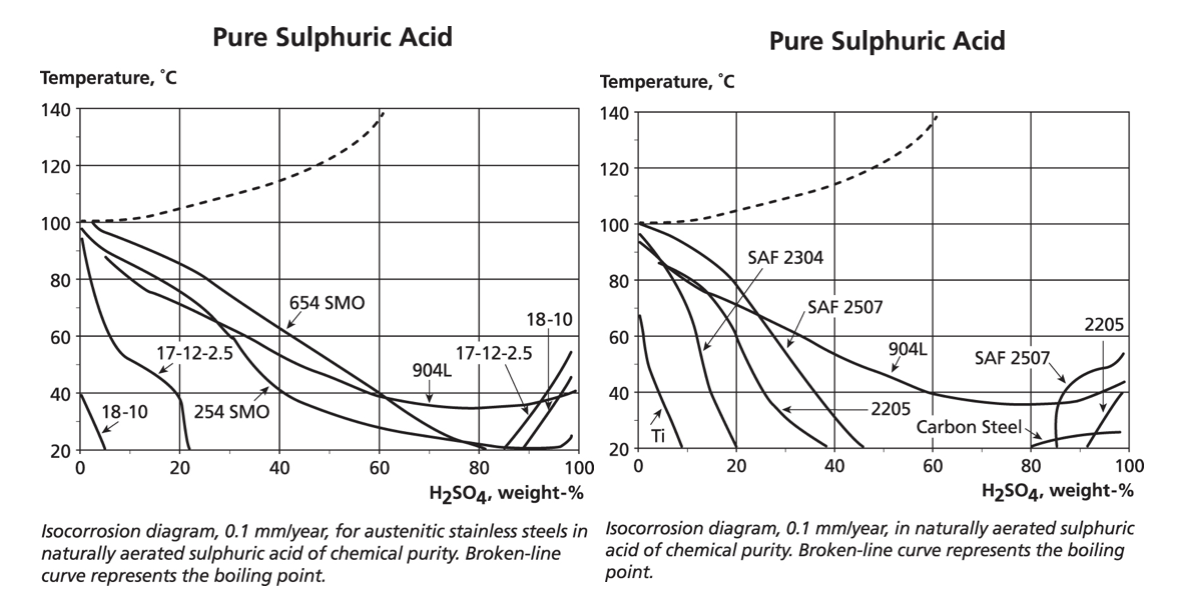
WHAT ABOUT IMPURITIES OR ADDITIVES?
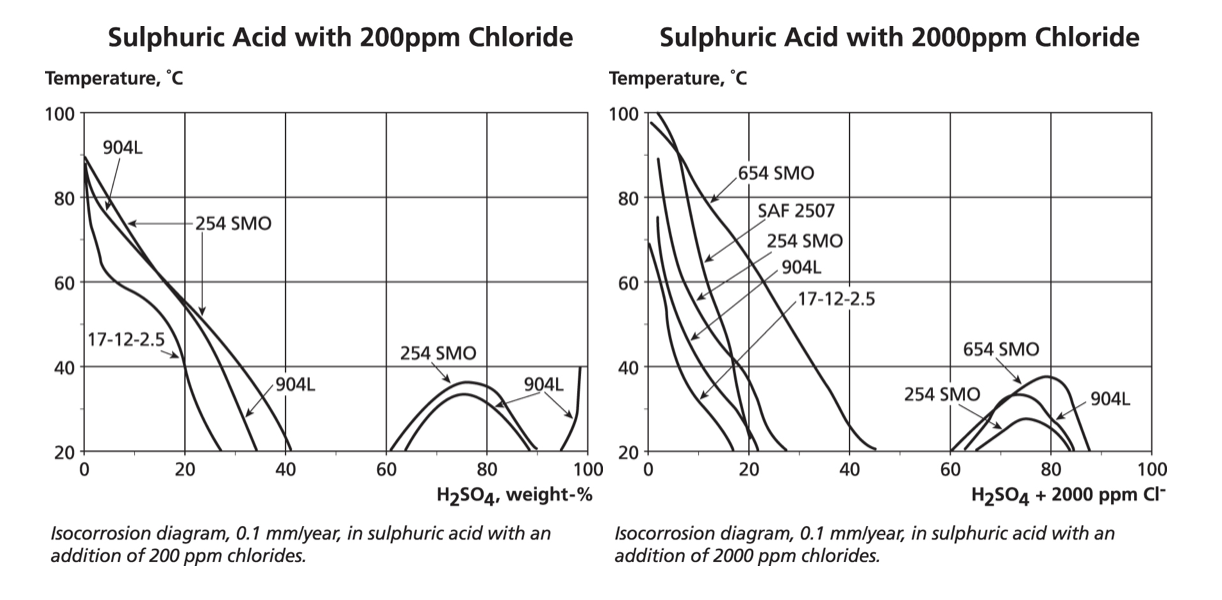
The graphs below show (and note the temperature scale changes from earlier graphs) the dramatic reduction in corrosion resistance when 200 mg/L of chlorides are added to sulphuric acid or ten times that amount, i.e. 2,000 mg/L. The heavily reducing range from about 40% to 60% acid concentration defeats even the high nickel 904L and 254/654 grades.
Nevertheless, a number of grades are potentially suitable for concentrations below 20% sulphuric even with significant chlorides. However, the graphs also show that at the other end of the concentration scale, the oxidising conditions, which occur for sulphuric acid above about 90%, are extremely aggressive if the acid is impure.
Some additives act as inhibitors to corrosion and this can be critical in selecting suitable materials for mineral extraction processes. For example, the graph below shows that adding iron ions to sulphuric acid improves the resistance of 316. Adding oxidising cupric ions has a similar effect but as with any inhibitor, attack can occur in crevices where the inhibitors may be used up. And despite the requirement for oxidising conditions to ensure stability of the stainless steel’s passive layer, it is possible to add too much oxidant as shown by the positive effect of small additions of chromic acid followed by a reduction in corrosion resistance if more chromic acid is added. It is relatively common to refer to the redox potential (rather than concentrations of oxidising ions) if the chemistry is not simple.
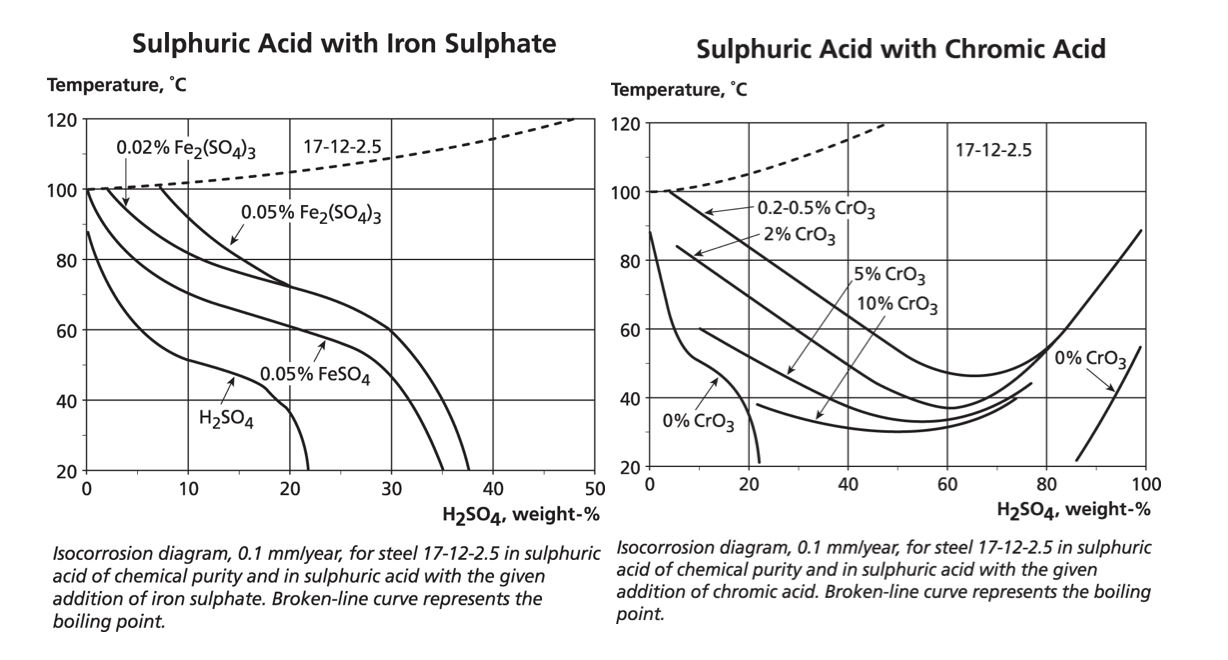
The data in this section is intended to show that while these iso-corrosion graphs are useful in predicting corrosion rates for specific pure compounds, the addition of aggressive ions, oxidisers or crevice conditions require more detailed consideration.
MATERIALS SELECTION FOR OTHER CHEMICALS
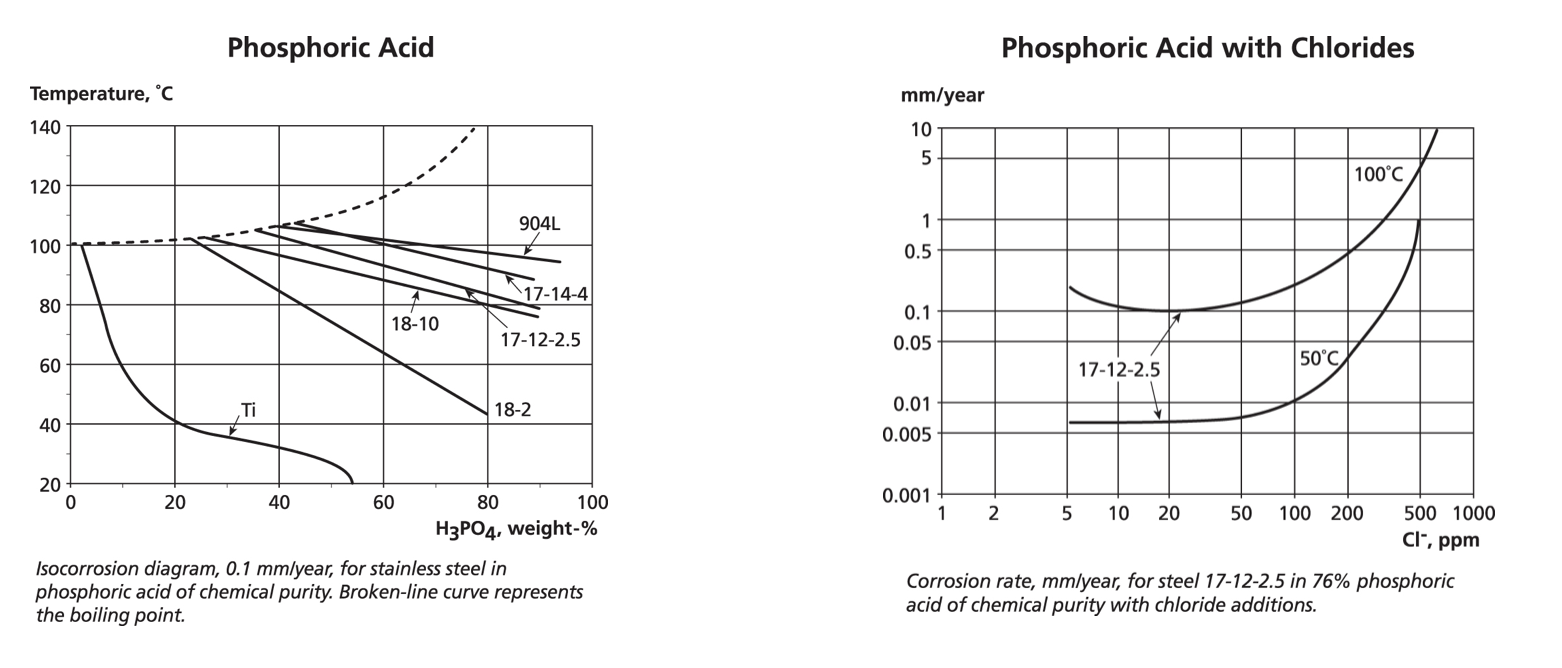
A very common chemical is phosphoric acid, which is used in cleaning, pre-treatments, food preparation and a host of other applications. It requires increasing chemical resistance with high temperatures and concentrations. For pure phosphoric acid, the iso-corrosion curves show a progression from ferritic 444, through the austenitic 304, 316, 317 to 904L. This is not an oxidising acid so although it removes iron contamination, it does not strengthen the passive film on stainless steels.
Phosphoric acid is frequently associated with chloride or fluoride ions especially in production from rock phosphate. The variation in composition in this wet process acid (WPA) means that iso-corrosion plots are of limited use. However, with thermally produced acid and various impurities, a plot of corrosion rate vs. contaminant ion concentration may be used instead of an iso-corrosion graph – in this case chlorides with the 2.5% molybdenum version of 316. This data is for exposure 24 hours a day, 365 days a year. Note that while the two graphs do not overlap, the trends of these different experimental plots do not exactly match, i.e. iso-corrosion curves provide trend data and not precise values.
ACIDS FOR CLEANING STAINLESS STEELS
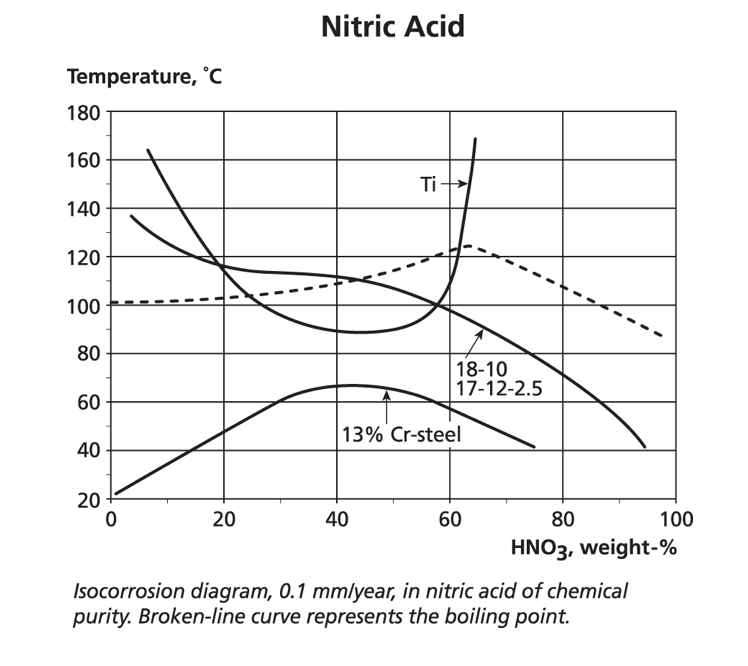
Both the chelating oxalic and citric acids, and the oxidising nitric acid, are widely used on stainless steels both for cleaning and passivation as shown in ASTM A380 and A967. Nitric acid can be used at elevated temperatures and low to medium concentrations without concern for the standard austenitics. However, at high concentrations and above ambient temperatures, they can suffer intergranular attack, unless a low carbon grade is used. In the same environment, molybdenum-containing grades may suffer intergranular attack of the intermetallic phases such as sigma.
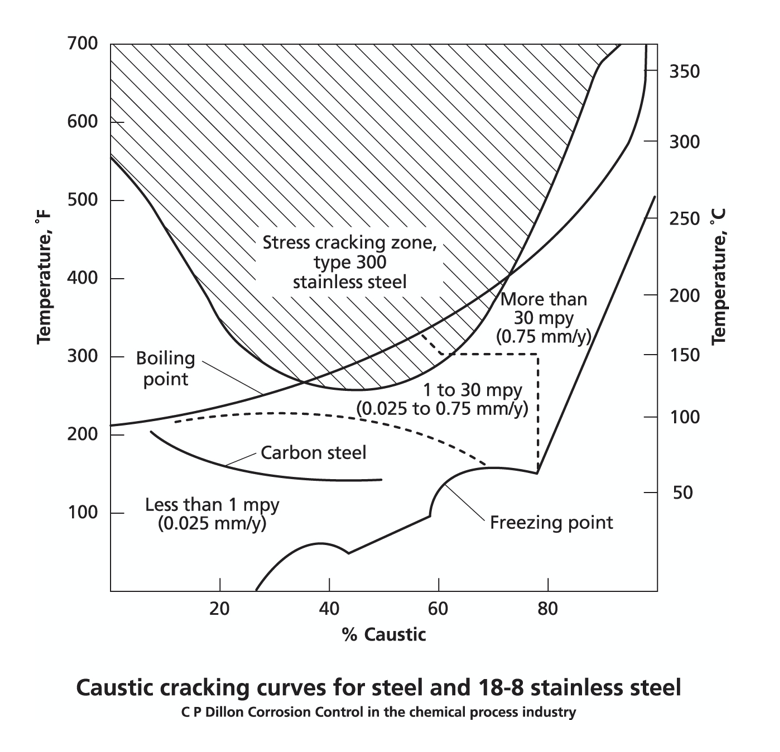
ALKALIS
If you intend to use a stainless steel with a new, relatively pure chemical, iso-corrosion curves offer an initial guide to the temperature and concentration limits against general attack. If there are contaminants or oxidants present, then the corrosion susceptibility can increase or decrease significantly and specialist advice should be obtained.
SUMMARY
If you intend to use a stainless steel with a new, relatively pure chemical, iso-corrosion curves offer an initial guide to the temperature and concentration limits against general attack. If there are contaminants or oxidants present, then the corrosion susceptibility can increase or decrease significantly and specialist advice should be obtained.
IMPORTANT DISCLAIMER: The technical recommendations contained in this article are necessarily of a general nature and should not be relied on for specific applications without first securing competent advice. Whilst ASSDA has taken all reasonable steps to ensure the information contained herein is accurate and current, ASSDA does not warrant the accuracy or completeness of the information and does not accept liability for errors or omissions.